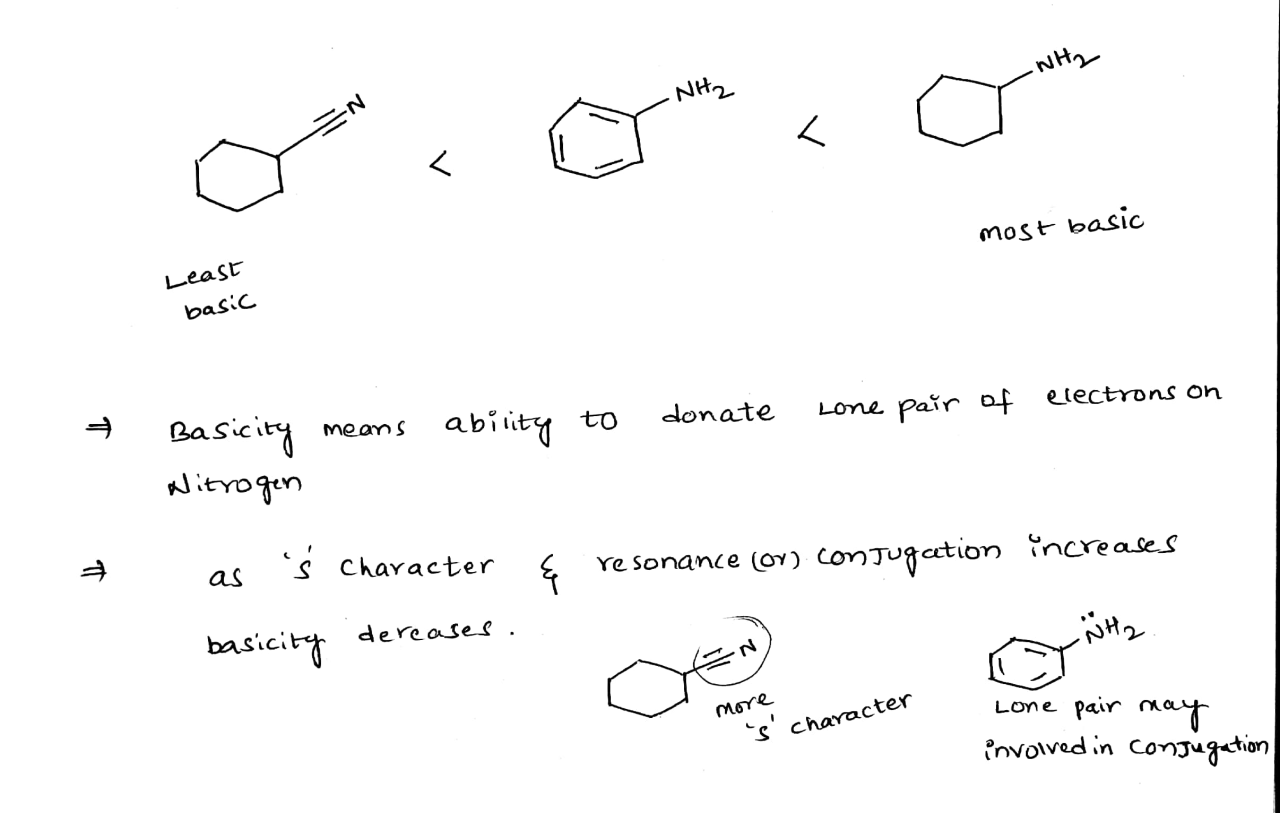
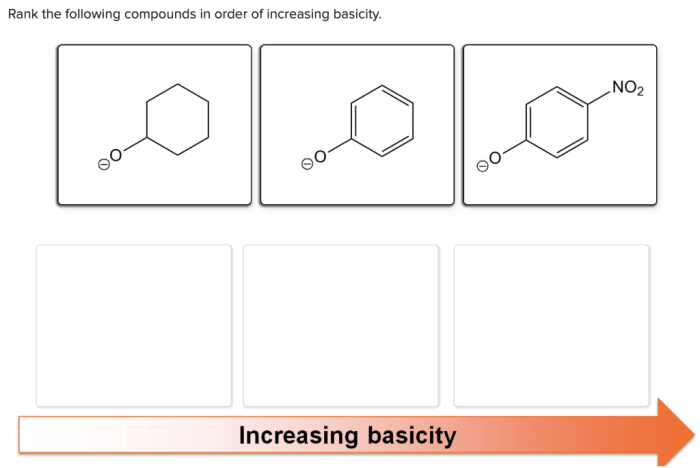
Rank the following anions in terms of increasing basicity: Hydroxide (OH-), Acetate (CH3COO-), Chloride (Cl-), Nitrate (NO3-), and Sulfate (SO42-). This ranking provides crucial insights into the chemical behavior and reactivity of these anions, enabling a deeper understanding of their applications in various fields.
Basicity, a fundamental concept in chemistry, plays a pivotal role in determining the strength of a base. By examining the factors influencing basicity, such as electronegativity, charge, and size, we can establish a clear understanding of the relative strengths of these anions.
Factors Affecting Basicity: Rank The Following Anions In Terms Of Increasing Basicity
The basicity of an anion is influenced by several factors, including:
- Electronegativity:The more electronegative the atom that forms the anion, the weaker the base. This is because electronegative atoms have a strong pull on electrons, making it less likely for the anion to donate electrons.
- Charge:The more negative the charge on the anion, the stronger the base. This is because anions with a greater negative charge have a stronger attraction for protons.
- Size:The larger the anion, the weaker the base. This is because larger anions have a greater electron cloud, which makes it more difficult for the anion to donate electrons.
FAQ Insights
What is the significance of basicity in chemistry?
Basicity is a crucial property that determines the strength of a base. It influences the reactivity of anions in chemical reactions and plays a vital role in various fields, including acid-base chemistry, biochemistry, and environmental science.
How do electronegativity, charge, and size affect basicity?
Electronegativity, charge, and size are key factors that influence the basicity of anions. Electronegative atoms tend to attract electrons more strongly, reducing the basicity of the anion. Conversely, anions with a higher charge and larger size are generally more basic.
What are the practical applications of basicity?
Basicity finds applications in diverse fields such as:
- Acid-base neutralization reactions
- Buffer solutions
- Catalysis
- Electrochemistry
- Environmental remediation