Label the following multi step reaction energy diagram – Label the following multi-step reaction energy diagram: this directive marks the commencement of a comprehensive exploration into the intricacies of multi-step reaction energy diagrams. These diagrams serve as invaluable tools for visualizing and analyzing the energetic landscape of complex chemical reactions, providing insights into their mechanisms, rates, and outcomes.
In this guide, we will delve into the intricacies of multi-step reaction energy diagrams, examining their components, interpreting their profiles, and exploring their applications in unraveling the mysteries of chemical reactions.
Multi-Step Reaction Energy Diagram
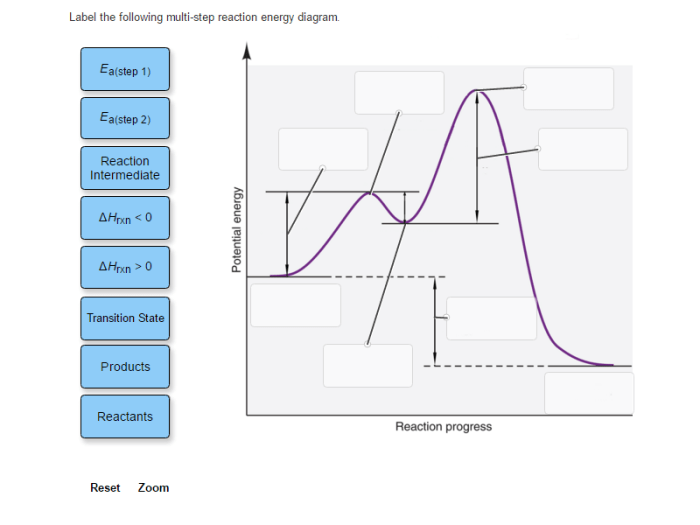
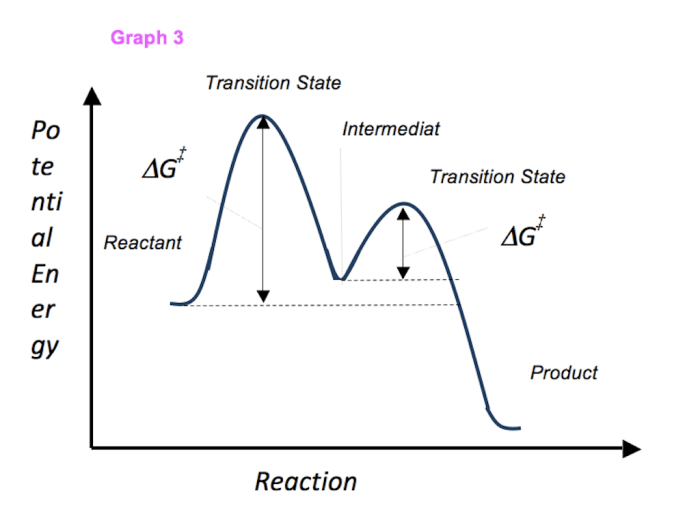
A multi-step reaction energy diagram is a graphical representation of the energy changes that occur during a multi-step reaction. It shows the energy of the reactants, products, transition states, and intermediates involved in the reaction.
The diagram is typically drawn with the reaction progress on the x-axis and the energy on the y-axis. The reactants are represented by the leftmost point on the diagram, and the products are represented by the rightmost point. The transition states are represented by the highest points on the diagram, and the intermediates are represented by the lower points.
Labeling the Energy Diagram
The following table lists the labels that are typically used to label a multi-step reaction energy diagram:
| Label | Description |
|---|---|
| Reactants | The starting materials of the reaction |
| Products | The final products of the reaction |
| Transition states | The highest energy points on the diagram, representing the unstable species that form during the reaction |
| Intermediates | The lower energy points on the diagram, representing the stable species that form during the reaction |
| Activation energy | The energy difference between the reactants and the transition state |
| Enthalpy change | The energy difference between the reactants and the products |
Analyzing the Energy Profile
The energy profile of a multi-step reaction can be used to determine the rate-determining step. The rate-determining step is the slowest step in the reaction, and it is the step that determines the overall rate of the reaction.
The activation energy of a reaction is the energy difference between the reactants and the transition state. The higher the activation energy, the slower the reaction rate. The enthalpy change of a reaction is the energy difference between the reactants and the products.
A positive enthalpy change indicates that the reaction is endothermic, while a negative enthalpy change indicates that the reaction is exothermic.
Applications of Energy Diagrams, Label the following multi step reaction energy diagram
Energy diagrams are used in chemistry to understand reaction mechanisms. They can be used to predict the products of a reaction, and they can be used to determine the rate-determining step. Energy diagrams are also used to design catalysts, which are substances that can speed up the rate of a reaction.
Key Questions Answered: Label The Following Multi Step Reaction Energy Diagram
What is a multi-step reaction energy diagram?
A multi-step reaction energy diagram is a graphical representation of the energy changes that occur during a multi-step chemical reaction. It shows the energy of the reactants, products, transition states, and intermediates involved in the reaction.
How can I use a multi-step reaction energy diagram to determine the rate-determining step?
The rate-determining step is the step with the highest activation energy. The activation energy is the energy barrier that must be overcome for the reaction to proceed.
What are some applications of multi-step reaction energy diagrams?
Multi-step reaction energy diagrams are used in a variety of applications, including:
- Understanding reaction mechanisms
- Predicting the products of a reaction
- Designing new catalysts